October 27, 2020
Variation in the Mineral Forms of Iron Oxide Affects Mobility of Contaminants
The fate of plutonium during transformation of poorly crystalline ferrihydrite to crystalline goethite revealed at the molecular scale.
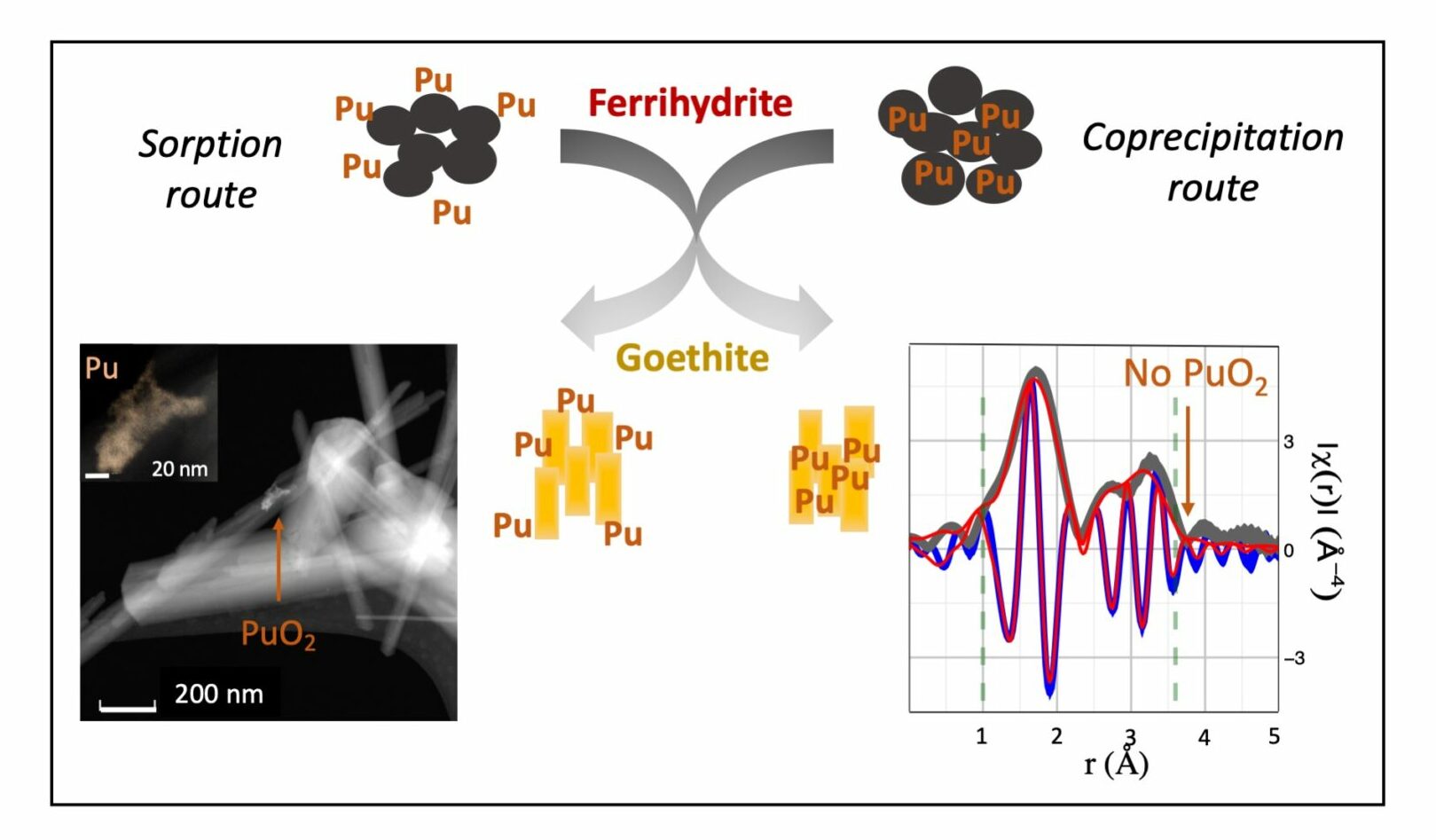
Ferrihydrite was synthesized with plutonium following either a sorption or coprecipitation process. The molecular-level structure of plutonium during ferrihydrite transformation to goethite will impact its long-term behavior in the environment.
[Reprinted with permission from Balboni, E., et al. "Transformation of Ferrihydrite to Goethite and the Fate of Plutonium." ACS Earth and Space Chemistry 4 (11), 1993–2006 (2020). DOI:10.1021/acsearthspacechem.0c00195. Copyright 2023 American Chemical Society.]
The Science
Plutonium contamination in the environment threatens water quality and human health. Once released into the environment, plutonium interacts with groundwater, minerals, microbes, and soil. Plutonium and many other heavy metals have a particularly strong affinity for iron oxide minerals. These iron oxide minerals are commonly subject to dissolution and transformation reactions in the environment, and the fate of plutonium during these processes is currently unknown. A multi-institutional team of scientists has recently demonstrated that these transformation reactions will impact plutonium’s molecular-level structure and its subsequent mobility.
The Impact
Iron minerals in transient and dynamic (bio)geochemical settings, such as sediment deposits in lakes or ponds, are subject to dissolution and phase transformation reactions, and the fate of sorbed species of contaminants during these processes is currently unknown. For example, iron oxide transformation reactions are expected to affect the molecular-level structure of plutonium associated with these important minerals. These transformation reactions may determine the long-term fate of plutonium in contaminated environments (e.g., contaminated soils and sediments) and engineered environments (e.g., underground nuclear waste repositories). Moreover, these same reactions are likely to play an important role in the mobility, cycling, and availability of other heavy metals and radionuclides in the environment.
Summary
The production and testing of nuclear weapons, nuclear accidents, and authorized discharges of radioactive effluents have contributed significantly to plutonium released into the environment. Once released, plutonium has a particularly high affinity for iron oxide minerals, which are common in soils and sediments. These iron oxide minerals are subject to dissolution and phase transformation reactions, but the fate of plutonium during these transformations is not well understood. In laboratory experiments, a multi-institutional team of scientists synthesized an amorphous iron mineral (ferrihydrite) with varying quantities of plutonium, following either a sorption or coprecipitation process. The ferrihydrite was then aged hydrothermally to yield a crystalline product (goethite). This is a common reaction process in nature. In samples prepared following the sorption method, plutonium was identified both as PuO2 precipitate and as a surface complex. For the samples prepared via coprecipitation, no PuO2 formation in the ferrihydrite precursor or in the low plutonium concentration goethite was observed. In these coprecipitation products, plutonium was found to be strongly bound to the minerals through either formation of an inner sphere complex, or an incorporation process. The results indicate that iron oxide transformation reactions will affect the molecular-level structure of plutonium associated with these important minerals. These same reactions are likely to play an important role in the mobility, cycling, and availability of other heavy metals and radionuclides in the environment.
Principal Investigator
Mavrik Zavarin
Lawrence Livermore National Laboratory
Program Manager
Paul Bayer
U.S. Department of Energy, Biological and Environmental Research (SC-33)
Environmental System Science
paul.bayer@science.doe.gov
Funding
This research was conducted at Lawrence Livermore National Laboratory (LLNL) under contract DE-AC52-07NA27344. It was supported by the Spent Fuel and Waste Science and Technology campaign of the U.S. Department of Energy’s (DOE) Nuclear Energy Program and the Environmental System Science (ESS) program within DOE’s Biological and Environmental Research (BER) Program.
References
Balboni, E., et al. "Transformation of Ferrihydrite to Goethite and the Fate of Plutonium." ACS Earth and Space Chemistry 4 (11), 1993–2006 (2020). https://doi.org/10.1021/acsearthspacechem.0c00195.

