April 12, 2024
How Soil Incubation Methods Affect Inferred Methane Production Temperature Sensitivity
A wide range of inferred Q10 values (1.2 to 3.5) is attributed to incubation temperatures, incubation duration, storage duration, and sampling time.
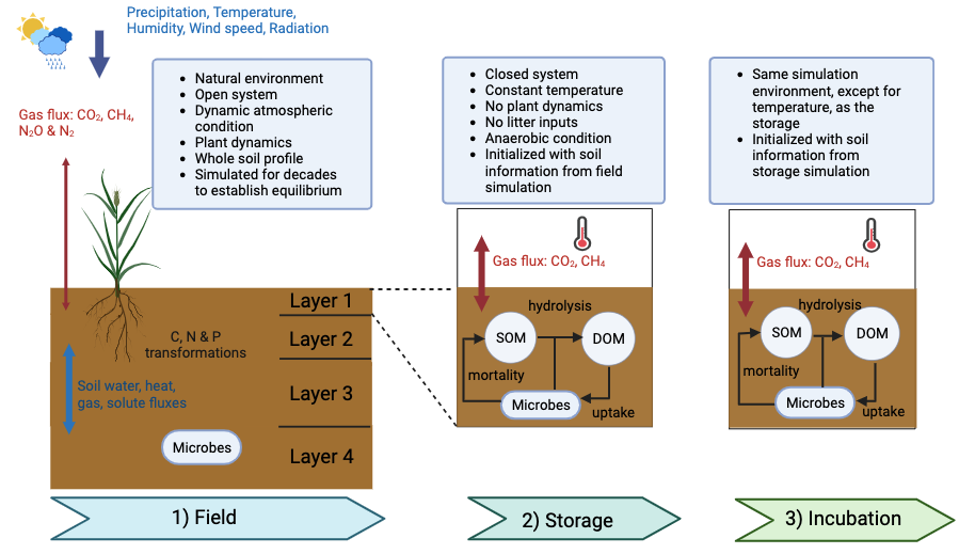
A schematic representation of the three-phase Field-Storage-Incubation (FSI) modeling approach.
[Reprinted under a Creative Commons Attribution 4.0 International License (CC BY 4.0) from Li, Z., et al. "Soil Incubation Methods Lead to Large Differences in Inferred Methane Production Temperature Sensitivity." Environmental Research Letters 19, 044069 (2024). DOI:10.1088.1748-9326/ad3565.]
The Science
Methane (CH4) is the second most important greenhouse gas after carbon dioxide. Quantifying how CH4 production changes with temperature is crucial to predicting how wetland ecosystems will respond to climate warming. Temperature sensitivity is often derived from laboratory incubation studies. This study applies observations and a well-tested model to interpret laboratory incubation observations. The findings explain how the inferred temperature sensitivity of CH4 production is affected by incubation duration, incubation temperatures, storage duration, storage temperature, and sampling time.
The Impact
Understanding and quantifying CH4 production temperature sensitivity is important to improving predictions of how wetland ecosystems will respond to and feed back to climate warming. The sensitivity of CH4 production to temperature is often described by a Q10 value. This study demonstrates that Q10 values of CH4 production and emission are regulated by a complex interplay of biological, biochemical, and physical processes. This interaction leads to the aggregated Q10 differing from those of the component processes. Terrestrial ecosystem models relying on a constant Q10 value to characterize temperature responses may therefore predict biased soil carbon cycling under future climate scenarios.
Summary
Researchers apply observations in a thawing permafrost peatland and a well-tested, process-rich model (ecosys) to interpret incubation observations and investigate controls on inferred CH4 production temperature sensitivity. Results show dynamic CH4 production rates are regulated by the interplay between substrates (dissolved organic carbon, acetate, and hydrogen) and activities of methanogens and fermenters. Seasonal variation in substrate availability and active microbial biomass of key microbial functional groups led to strong time-of-sampling impacts on CH4 production. CH4 production is higher with less perturbation post-sampling, i.e., shorter storage duration and lower storage temperature. This study reports a wide range of inferred Q10 values (1.2 to 3.5), which is attributed to incubation temperatures, incubation duration, storage duration, and sampling time.
The Field-Storage-Incubation (FSI) framework for simulating incubations provides valuable insights for interpreting incubation observations. Further, this work emphasizes the need to accurately measure important variables such as substrate availability and active microbial biomass during incubation experiments to improve mechanistic understanding of carbon cycling responses to warming.
Principal Investigator
William J. Riley
Lawrence Berkeley National Laboratory
[email protected]
Program Manager
Daniel Stover
U.S. Department of Energy, Biological and Environmental Research (SC-33)
Environmental System Science
[email protected]
Funding
This research is a contribution of the EMERGE Biology Integration Institute, funded by the National Science Foundation (NSF), Biology Integration Institutes Program, award no. 2022070.
Additional support for individual contributors included the following: W. J. R. was supported by the Belowground Biogeochemistry Scientific Focus Area and Z. A. M. was funded by the RUBISCO Scientific Focus Area, both funded by the U.S. Department of Energy (DOE), Office of Science, Biological and Environmental Research program (BER) under contract no. DE-AC02-05CH11231. J. T. was supported by the Laboratory Directed Research and Development Program of Lawrence Berkeley National Laboratory (LBNL). R. K. V. was supported by DOE Grant (DE-SC0023456).
The research team thanks the Swedish Polar Research Secretariat and SITES for the support of the work done at the Abisko Scientific Research Station. SITES is supported by the Swedish Research Council’s grant 4.3-2021-00164. Autochamber measurements between 2013 and 2017 were supported by a grant from a NSF MacroSystems program (NSF EF 1241037, R. K. V.). This research used the Lawrencium computational cluster resource provided by the IT Division at the LBNL (supported by the Basic Energy Sciences program of DOE’s Office of Science under contract no. DE-AC02-05CH11231). Incubation and field observation data were collected under the IsoGenie Project, funded by BER’s Genomic Science Program, grant nos. DE-SC0004632, DE-SC0010580, and DE-SC0016440.
References
Li, Z., et al. "Soil Incubation Methods Lead to Large Differences in Inferred Methane Production Temperature Sensitivity." Environmental Research Letters 19 044069 (2024). https://doi.org/10.1088/1748-9326/ad3565.

