April 26, 2021
Particulate Organic Matter Controls Lead Release During Redox Cycles in Floodplain Soils
Lead (Pb) released from mineral phases during hydrologic redox cycles is retained by particulate organic matter, limiting dissolved Pb concentrations
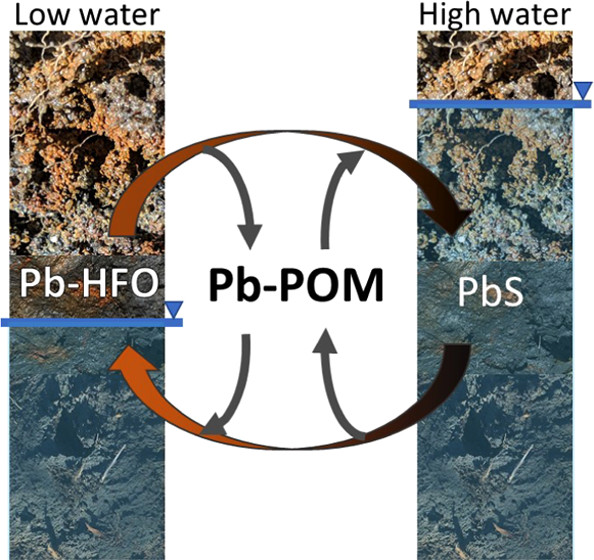
Dynamic hydrology drives redox changes in floodplain soils, dissolving and reprecipitating Lead(II) sulfide (PbS) and lead (Pb) adsorbed on Fe(III)-(hydr)oxides. Particulate organic matter retains released Pb prior to reprecipitation, limiting dissolved Pb concentrations.
[Reprinted with permission from Dewey, C., et al. “Porewater Lead Concentrations Limited by Particulate Organic Matter Coupled With Ephemeral Iron(III) and Sulfide Phases during Redox Cycles Within Contaminated Floodplain Soils.” Environmental Science and Technology 55(9), 5878–5886 (2021). Copyright 2021 American Chemical Society.]
The Science
Lead (Pb) contamination in soils is a major threat to water quality. Although Pb tends to occur in sparingly soluble minerals, changes in dissolved oxygen concentrations can promote dissolution of these minerals, potentially causing spikes in dissolved Pb concentrations and transport of dissolved Pb to drinking water sources. Researchers examined the fate of Pb during changes in oxygen concentrations in contaminated floodplain soils and found that Pb released from mineral phases is retained by particulate organic matter (POM). Thus, POM limits spikes in dissolved Pb concentrations and prevents transport of dissolved Pb.
The Impact
Lead is highly toxic, and its consumption in any amount is considered unsafe. As a result of mining activities and leaded gasoline, soil lead contamination is widespread. It is critical to understand the fate of Pb in soils to assess the risks it presents to freshwater quality. Dissolved Pb is particularly dangerous, as it is easily transported and consumed. The research findings reveal that although common solid Pb phases are dissolved during changes in water levels in floodplain soils, released Pb is immediately retained on particulate organic matter, and dissolved Pb remains low. Thus, although the soils studied contain appreciable Pb, it likely does not pose a threat water quality in dissolved form.
Summary
Objectives were to resolve Pb speciation and partitioning across hydrologically controlled redox transitions and to determine the extent of Pb release during these transitions. To examine the effects of soil redox transitions on Pb partitioning, researchers tracked solid-phase Pb speciation and dissolved Pb concentrations in mining-affected floodplain soils near Crested Butte, CO. Groundwater levels at the study site varied seasonally, driving changes in soil redox conditions. The team collected depth-resolved soil and porewater samples at 2 – 4 week intervals between June 2 and October 26, 2018, while monitoring groundwater levels hourly. Findings determined solid phase Pb speciation using Pb L3-edge extended X-ray absorption fine structure (EXAFS) measurements. When water levels were high in June and early July, iron- and sulfate-reducing conditions developed in the soils—dissolving Fe(III)-(hydr)oxides, releasing associated Pb, and promoting PbS formation. As water levels declined into August, oxygen was reintroduced to the soil profile, and Fe(III)-(hydr)oxides precipitated while PbS was dissolved. A beaver dam was built near the site in late August, which caused water levels to rise again, resulting in Fe reducing conditions. As reducing conditions transitioned to oxidizing conditions and vice versa, researchers observed an increase in Pb adsorbed on particulate organic matter. They also did not observe increases in dissolved Pb concentrations. Taken together, this indicates that particulate organic matter retains Pb released during dissolution of Fe(III)-(hydr)oxides and PbS, thereby limiting its dissolved concentrations in porewater.
Principal Investigator
Christian Dewey
Stanford University
[email protected]
Funding
This research was supported by the SLAC Groundwater Quality SFA program in the Office of Biological and Environmental Research’s (BER) Environmental System Science (ESS) program (formerly Subsurface Biogeochemistry Research) within the U.S. Department of Energy’s Office of Science and by the SBR Project Award Number DE-SC0016544. Use of the Stanford Synchrotron Radiation Lightsource and SLAC National Accelerator Laboratory is supported by the Office of Basic Energy Sciences (BES) in DOE’s Office of Science, under contract No. DE-AC0276SF00515.
References
Dewey, C. et al. "Porewater Lead Concentrations Limited by Particulate Organic Matter Coupled with Ephemeral Iron (III) and Sulfide Phases during Redox Cycles within Contaminated Floodplain Sediments." Environmental Science and Technology 55 (9), 5878-5886 (2021). https://doi.org/10.1021/acs.est.0c08162.

